Research Ethics Committee
The primary purpose of the JMCFI-Research Ethics Committee is to safeguard the rights, well-being, and dignity of research participants while promoting the highest standards of research integrity.
Research Ethics
Committee
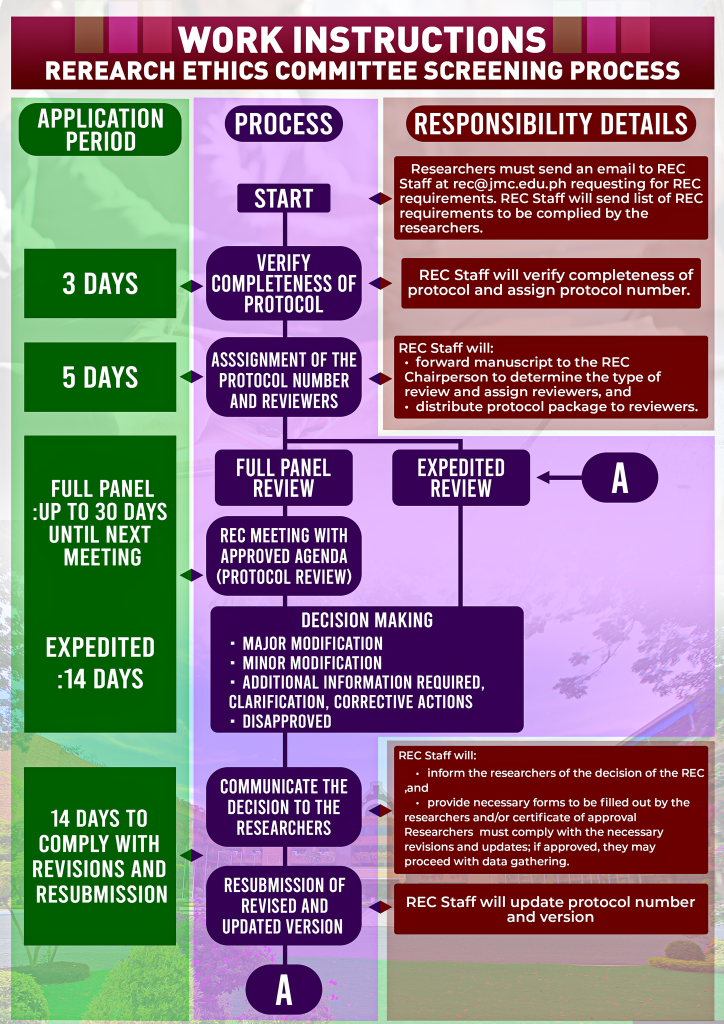
REC Process Flow
REC List of Requirements

Research Ethics Committee
- Application Form (REC Form 61)
- Letter Request for Review (REC Form 3,6, signed by the adviser/dean)
- Endorsement/Referral Letter (REC Form 3.7, signed by the adviser/dean)
- Full Proposal/Study Protocol (revised manuscript based on your proposal defense
- Certificate of Proposal Defense (c/o Research Dept.)
- Technical Review Approval (Routing Form) – (c/o Research Dept.)
- Curriculum Vitae of Lead Researcher/s
- Confidentiality Agreement (REC Form 41)
- Budget (REC Form 6.13)
- GANTT Chart (REC Form 6.14)
- Dummy Table of Results (REC Form 6.15)
- Informed Consent Form (English, Filipino, or others) (REC Form 5.5) = Assent Form (if applicable – il your participoits are at minor age)
- Receipt of Payment – please proceed to the REC Office in Room 224 for payment. if you are from College of Medicine (COM), disregard this one. This will be part of your miscellaneous fee.
| for undergrad | Php 3,000.0 |
| For College of Medicine (COM) | Php 4,000.0 (covered in Miscellaneous) |
****** Please Note Please designate one representative from your group to handle all communication with the REC. This person will be responsible for emailing requirements, processes, and other protocols-related documents throughout the entire process. This is for REC to easily track protocols-related documents of submissions in the database.
REC FORMS
Other Pertinent Documents

Research Ethics Committee
Below are some responsibilities after approval
- Submit Protocol Amendments (REC Form 6.4) (if any) for JMCFI REC approval before implementing them;
- Amendments – refer to any change or revision in the protocol made after its approval from REC
- Submit on-site Serious Adverse Events (SAEs) (REC Form 6.8) and Suspected Unexpected Serious Adverse Reaction (SUSAR) reports (if applicable) to the JMCFI-REC within the day of its detection;
- Submit Progress Report (REC Form 6.5) as determined by the REC based on the level of risk; •
- Submit Final Report (REC Form 6.12) after completion of protocol procedures at the study site;
- Report Protocol Deviations/Violations (REC Form 6.6)
- Protocol Deviation – refers to a non-compliance with the approved protocol that does not increase risk or decrease benefit to participants or does not significantly affect their rights, safety or welfare or the integrity of data.
- Example: missed visit, non-submission of a food diary on time.
- Protocol Violation – refers to a non-compliance with the approved protocol that increases risk or decreases benefit to participants or significantly affects their rights, safety or welfare or the integrity of data.
- Example: incorrect treatment, non-compliance with inclusion/exclusion criteria.
- Comply with all relevant international and national guidelines and regulations;
- Abide by the principles of Good Research Practice and Ethical Research.
CONNECT with US
Subscribe to our newsletter to hear the latest news



